Those who are held accountable for what happens in healthcare – whether a physician, care team member, pharmaceutical company, medical device manufacturer, or health system – need to understand what happens, why it happens, and how to improve.
But there’s a gap between what really happens in healthcare and what is “supposed” to happen. Clinical research, guidelines, and case studies tell us what is “supposed” to happen. Real-world data from the clinical setting or the patient themselves tells us what actually happened.
And it’s what actually happens that really matters.
Using Clinical Data to Fill the Medical Device Knowledge Gap
Addressing this gap requires collecting real-world data, transforming it into real-world evidence (RWE), and sharing it with those who can use it to advance care.
We can illustrate this with a relevant clinical example.
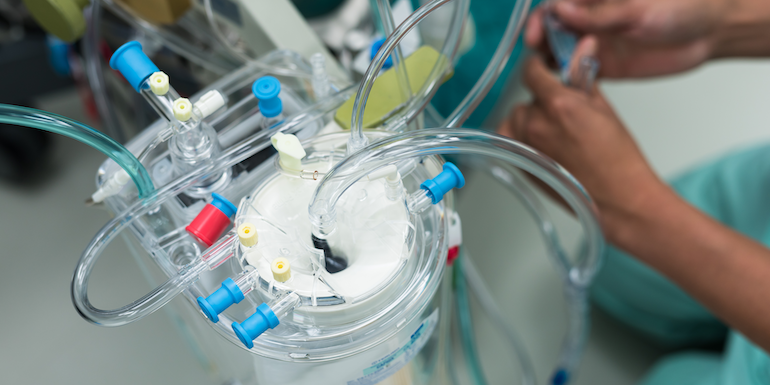
Extracorporeal membrane oxygenation, commonly referred to as ECMO, is a treatment that uses an artificial heart and lung to support the body when a person’s own organs are too sick to do the job. ECMO itself will not cure a patient’s heart or lungs, but it buys time for patients to heal or for alternative treatments to be coordinated in cases of severe cardiac or respiratory failure.
Knowing what truly happens in clinical settings – having real-world evidence – is especially important here.
Physicians and ECMO practitioners need to have reliable data on how and when it is safe and effective to put patients on ECMO. But they are only part of the equation.
Given the complexity of care and the high rate of mortality and complications, ECMO care presents limitations for traditional clinical trials. This means despite decades of widespread use, until recently, ECMO has been largely performed off-label in the United States.
This is a crucial clinical knowledge gap for the companies manufacturing devices used in ECMO. They know anecdotally that their devices are being used in this way, but they do not have access to any data demonstrating the safety and effectiveness of their devices used in a clinical context.
Clinical Quality Registry for Physicians
To solve for their data and knowledge needs, physicians from across the globe have come together as part of the Extracorporeal Life Support Organization (ELSO). They collaborate, share data, and learn from one another through the ELSO Quality Registry.
The registry tracks use of medical devices for ECMO and collects real-world data on the patients who have received ECMO. It contains details on patient and device demographics, clinical information, patient outcomes, and adverse events. ELSO Registry data has been used to:
- Investigate high and low performing centers.
- Establish clinical best practice guidelines.
- Publish a wealth of detailed peer-reviewed research.
- Demonstrate the safety and effectiveness of current ECMO treatments.
- Improve when and how ECMO is provided in the future.
Medical Device Registry for Manufacturers
To help manufacturers fill their knowledge gap, ELSO leveraged its clinical Quality Registry to share data through a medical device registry.
The device registry, ELSO Evidence Explorer, takes real-time data from more than 700 centers worldwide and transforms it to help manufacturers know how clinicians are actually using their devices, what the average patient looks like, and how their device truly performs in the real-world.
Manufacturers leverage this data and review the usage, the incidence of mortality, and ECMO-specific complications where their devices were used as part of the circuit.
Using Registry Data for Real-World Evidence
ELSO’s long-standing registry is a rich data source with a lot of value. But it wasn’t enough to simply provide raw data exports to manufacturers. Creating a meaningful medical device registry required a few key steps.
1. Post-Market Surveillance of an Evolving Market
One of the greatest advantages of real-world registry data – that it is longitudinal – also presents unique challenges. Historical case data collected by ELSO provides invaluable benchmark information for current and new devices.
But this also means ELSO must continually work with industry stakeholders to make sure the devices being tracked within its registry accurately reflect an evolving market. As new devices and models hit the market, older devices are retired or devices and manufacturers are acquired, ELSO ensures registry data appropriately reflects these changes.
2. Conducting Data Discovery
ELSO conducted a data assessment to identify what data in its registry would be valuable to manufacturers, assessing in particular those clinical situations where real-world registry data tracks either clinical practice or patient populations that cannot be easily studied via traditional trial-based means.
For reasons described above, ECMO cannot be studied via a clinical trial, making this assessment process straightforward for ELSO. Other medical societies and registries should consider where their registry data tracks populations that are or cannot be studied through clinical trials.
Additionally, registries tracking patients with chronic conditions and longitudinal treatments can re-survey specific patient cohorts through patient-reported outcomes surveys. Ultimately, ELSO worked with manufacturers and regulators to inform how their registry data could meet the needs of ECMO manufacturers, and what reports they could provide.
3. Assessing Data Quality
Data quality is important for any registry work. For ELSO, this starts with automated validation of each data element, ensuring only appropriate data can be submitted to the registry. Additionally, they conduct randomized site audits to compare registry data to corresponding EHRs.
Using registry data with key stakeholders, including the U.S. Food and Drug Administration (FDA) and manufacturers, requires additional effort and coordination. ELSO works with these stakeholders to ensure they understand how and where data is collected. Accurately tracking how data elements have been added and removed from the registry over time is of particular importance.
4. De-Identifying and Analyzing Data
ELSO Registry data is de-identified to protect the privacy of participating institutions and patients. An automated analytic process also cleanses and transforms the real-world data collected into statistically validated real-world evidence. This ensures data can be used effectively by manufacturers and regulators while protecting registry participants.
5. Designing Dynamic Web-Based Reporting
Participating manufacturers review and interact with this RWE through a dynamic, web-based reporting tool. They can build their own custom reports and queries in real time – all in a way that protects the identities of participating institutions, patients and industry competitors.
Sharing Real-World Evidence Improves Outcomes
The way ELSO uses its registry to bridge the medical device knowledge gap with RWE is powerful. Other medical specialties and patient registries can do the same. Sharing insights from these clinically-rich data assets is one of the key ways to advance care and improve health outcomes for all.
For more information on our real-world evidence and registry solutions, contact us at info@arbormetrix.com.
To learn more about ELSO Evidence Explorer, visit elso-explorer.arbormetrix.com.
