Trusted Real-World Evidence that Delivers Real-World Results
The Need for Real-World Evidence Is Growing.
ArborMetrix is positioned to tackle the challenges and needs of life sciences companies today.
- Lower Costs: Reduce the cost and increase ease of clinical trials
- Data Use: Increase and leverage Real World Data (RWD) use for multiple purposes- tracking patient safety, informing clinical trial/study design
- Understand Patient Populations: Better understand subpopulations including those affected by health disparities and heterogeneity of treatment effects.
- Data Integrity: The increasing use of Value/Outcomes Based Contracts by Life Science companies and payers is dependent on accessing reliable RWD

Our depth of clinical registry experience allows us to deliver unique partnerships and datasets across different stakeholders: medical specialty societies, provider organizations, medical device companies and pharmaceutical companies.
Our Proven technologies in data integration, Case Report Forms (CRFs), Patient Reported Outcomes (PROs), Electronic Health Record (EHR) integration, and clinical data registries gives you the irrefutable data and insights you need so you can be confident in meeting RWD requirements

Accelerate Your Path to Value
- Demonstrate device effectiveness in large, diverse patient groups.
- Identify risks, complications, and adverse outcomes as they arise.
- Compare new products with existing options and the standard of care.
- Update guidelines as certain populations find more or new benefits.
- Comply with regulatory requirements.

Accelerate Your Path to Value
- Demonstrate device effectiveness in large, diverse patient groups.
- Identify risks, complications, and adverse outcomes as they arise.
- Compare new products with existing options and the standard of care.
- Update guidelines as certain populations find more or new benefits.
- Comply with regulatory requirements.
Understand and Collect the Right Data to Drive Insights
Comprehensive acquisition of high-quality data is the foundation of success. Our philosophy of partnership pairs upfront definition and implementation of a robust data strategy and deep knowledge of measure development and outcomes across multiple clinical areas to ensure success and deliver actionable insights that drive results.
EXAMPLES OF DATA COLLECTED
- Adherence
- Adverse Events
- Co-morbidities
- Demographics
- Direct Streams from EHR
- Efficacy / Clinical Effectiveness
- Mortality
- Morbidity
- Patient Reported Outcomes / QoL
- Social Determinants of Health Data
- Supplemental Clinical Data with PRO
AREAS OF DEEP CLINICAL EXPERTISE
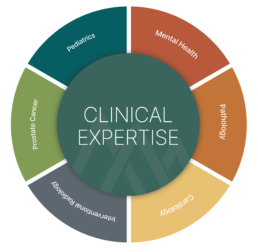
Transform Data into Evidence

ArborMetrix’s deep knowledge of measure development and outcomes across clinical areas ensures success. Leverage clinically rich, regulatory grade evidence to understand performance in real-world settings.
- Collect, aggregate, and validate large amounts of data from multiple sources with ease.
- Transform data into trusted evidence with proven methods and models.
- Explore device performance via intuitive real-time reports and tools.
Gain Flexibility and Control of Your RWE
Industry leading RWE engine, AMxCore, uses advanced statistical models for fair comparisons. We meet your needs with analytics technology that delivers the evidence you need when and how you need it, and helps you accelerate your path to value.
- Explore existing data and evidence.
- Understand utilization, complications, and outcomes.
- Identify areas for further research and improvement.

Demonstrate Safety and Drive Value

Expertise plus technology to build and unlock high quality data through patient registry partnerships ArborMetrix delivers exponential insights you can rely on to address your top priorities, deliver value, improve outcomes, and achieve results.
- Advance research and post-market surveillance across the product lifecycle.
- Support regulatory and payment strategy.
- Supplement clinical trial data.
Leverage Technology to Demonstrate Safety, Effectiveness, and Value
 Use high quality data to support clinical trials or post market surveillance studies. |
 Leverage research-grade evidence to accelerate clinical research and investigate trends and outcomes. |
 Advance care, reduce adverse events, and improve guideline adherence with evidence-based insights that drive improvement. |
 Inform a complete view of outcomes with statistically-valid patient data to support data-driven decision-making. |
Leverage Technology to Demonstrate Safety, Effectiveness, and Value
 Use high quality data to support clinical trials or post market surveillance studies. |
 Leverage research-grade evidence to accelerate clinical research and investigate trends and outcomes. |
 Advance care, reduce adverse events, and improve guideline adherence with evidence-based insights that drive improvement. |
 Inform a complete view of outcomes with statistically-valid patient data to support data-driven decision-making. |
Why It Matters
Registries Set The Foundation for Post-Market Surveillance
Patient registries are useful across many parts of healthcare. One of their most valuable purposes is post-market surveillance. Registries collect, analyze, and interpret large amounts of real-world data. This sets the foundation for successful post-market surveillance. When these three are done well, the result is a vast data resource that provides real-time, ongoing data collection, and is a central source of truth to answer key questions. Registries are used to support everything from registry-based studies and clinical trials to comprehensive post-market surveillance programs.
Life Sciences Resources
Insights To Support Your Improvement Journey
February 1, 2023
3 Things to Support Your Quality Program
July 14, 2021
What Is Quality Improvement in Healthcare?
March 31, 2021
How to Define Success for Your Clinical Registry
March 11, 2021








