Medical specialty societies were founded on two key principles: knowledge sharing and the exchange of clinical insights to improve medical practice. That central task has evolved as societies use clinical data registries to serve diverse and important stakeholders who are also involved in ensuring optimal health outcomes.
Medical specialty societies were founded on two key principles: knowledge sharing and the exchange of clinical insights to improve medical practice. That central task has evolved as societies use clinical data registries to serve diverse and important stakeholders who are also involved in ensuring optimal health outcomes.
These clinical data registry stakeholders include:
- Clinicians
- Health Systems, Hospitals, and Provider Organizations
- Patients
- Public and Private Payers, and
- Life Science Organizations (Pharmaceutical and medical device companies)
Societies and their registries are vehicles to further data-driven care through collaboration with these health care stakeholders. New opportunities frequently arise for registries to add unique value through the data insights they provide — especially through partnerships with life science organizations.
Specifically, there are four common ways for registries to empower life science organizations with new understandings in patient outcomes. These opportunities further support real-time insights in clinical care.
- Patient Cohort Analytics
- Post-Market Surveillance
- Clinical Decision Support
- Registry-Based Clinical Trials
Let’s examine each to understand their value to industry and value to your mission.
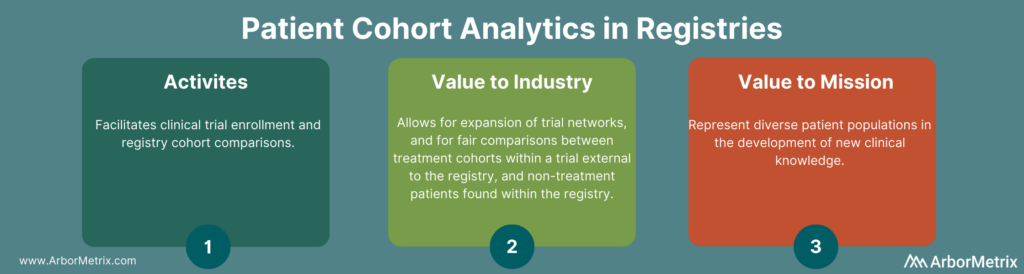
1. Patient Cohort Analytics and Registries
Patient cohort analytics are useful in several ways. They provide analytic tools to facilitate activities like clinical trial enrollment and registry cohort comparison for research.
Registries transform raw data into tools that provide tremendous value to trial sponsors in two ways:
- Identification of individual patients who match a specific cohort of interest and meet clinical trial inclusion and exclusion criteria. This allows trial sponsors to expand their trial network beyond standard academic centers and into community care settings, and through rapid enrollment of trial participants.
- Creation of comparison cohorts of registry patients through propensity-score matching. This allows trial sponsors to allow for fair and accurate comparison between treatment cohorts within a trial external to the registry and non-treatment patients found within the registry.
2. Post-Market Surveillance and Registries
Patient registries are uniquely useful in their ability to facilitate post-market surveillance for three reasons:
- They advance post-market surveillance activities to help us better understand the real-world safety and effectiveness of procedures, treatments, and devices.
- They solve complex problems for device and pharma companies. These organizations in general are faced with complex barriers when seeking real-world data for purposes of research and development, quality improvement, or regulatory requirements.
- They deliver the comprehensive technology needed to build a registry that collects real-world data, transform it into real-world evidence, and make it accessible and useful.
Simply stated, registries provide real-time answers to common post-market surveillance questions, like:
- How is a device or drug actually used in real-world settings?
- What does the average patient look like?
- How does a product truly perform?
3. Clinical Decision Support and Registries
Clinical decision support tools convert retrospective registry data into powerful point-of-care tools with immediate impact on patient care.
Decision support tools are built to take the guess work out of trade-off decisions between treatment courses. With real-world data and a user-friendly interface, decision support apps are valuable resources. They enable clinicians and patients to prioritize outcomes and establish appropriate expectations.
At the same time, decision support tools can be designed to demonstrate the degree to which device or pharmaceutical factors drive outcomes. These tools create significant value for industry stakeholders and set the stage for value-based reimbursement.
4. Clinical Trials and Registries
Registry-based clinical trials (RBCTs) set the gold standard for creating value for multiple stakeholders.
RBCTs rely on the registry’s network of participating clinicians, their patients, and the foundational registry dataset. They identify participating investigators and clinicians, facilitate patient enrollment, and support the majority of data elements required for trial completion. Registries also capture specialized clinical trial data through custom patient surveys, electronic case report forms, and data mapping.
The process ultimately leads to a clinical trial completion that is much quicker and more cost-effective than traditional methods.
 Registry Data Partnership Readiness
Registry Data Partnership Readiness
There are a few key steps to determining your organization’s readiness for registry data partnerships with industry.
- Understand the market opportunity. To design an effective program, conduct market research to understand who would find value in your registry data, what products or therapies they have on the market, and how those products are valued.
- Assess your registry data. You’ll need to know what data in your registry would be valuable to medical device manufacturers or pharmaceutical companies. Assess those clinical situations where real-world registry data tracks either clinical practice or patient populations that can’t be easily studied via traditional trials.
- Validate your registry data quality. Data quality is important for any registry work. Before initiating registry-based clinical trials or making de-identified registry data available to industry, you want to ensure the data quality is regulatory-grade. Achieving this requires a thorough data quality analysis to assess data integrity across dimensions such as completeness, concordance, precision, and currency.
Developing Registries for Many Stakeholders
Registries are powerful tools for effectively generating new clinical insights for key stakeholders and creating meaningful partnerships with life science companies.
These stakeholder collaborations use registries to employ valuable data tools to evaluate and improve patient outcomes, and in the process, help to further support the operations of registries and their underlying missions.
ArborMetrix offers an industry-leading, proven registry solution that meets the needs of medical device and pharma companies, medical specialty societies, and clinicians.