How to Define Success for Your Clinical Registry
 Medical specialty societies and patient foundations aim to improve healthcare. Clinical registries are one of the greatest instruments that guide how best to do that.
Medical specialty societies and patient foundations aim to improve healthcare. Clinical registries are one of the greatest instruments that guide how best to do that.
Clinical data registries are also called patient registries and disease registries. Professional medical specialty societies tend to use the term clinical data registry, while research and patient foundations and government organizations lean toward patient registry. Because disease registries are condition-specific, that term is often used among industry organizations.
A registry transforms real-world healthcare data into real-world evidence. The purpose is to evaluate and improve outcomes for a patient population defined by a particular condition, disease, or exposure.
Specifically, registries track information about the health status of patients and the care they receive. They bring together large data sets and analyze trends or patterns in treatments and outcomes to help inform best practices, guidelines, and treatment decisions.
Over the past 40 years, registries have become a central resource for medical societies, physicians, and, increasingly, for patients too. The majority of the 46 medical society members of the Council for Medical Specialty Societies (CMSS) manage patient registries. These groups make an aggregate investment of approximately $500 million over five years, according to CMSS. [1]
So what impact do these registries have on healthcare? How do you know if your registry is measuring up to what a modern registry is capable of achieving?
What Is a Successful Registry?
To put it simply, a successful registry measurably improves patient care.
It could be met through quality improvement programs, clinical research, regulatory reporting, or informing health policy decisions. It could be done using huge volumes of data from a large set of physicians, health systems, and practices — or from a small, yet statistically significant, group of targeted participants. Either way, the first step to measuring registry ROI is knowing what you want to achieve and identifying how, where, and when you will measure your own results.
What Are Key Metrics for Measuring Registry Success?
There are several key metrics you can use to determine your registry’s performance and value.
We separate these key metrics into performance metrics and results. Together, these tell a data-driven story of how well your registry is doing at achieving its objectives. They also provide useful data to inform future registry direction, enhancements, and changes.
Registry Success: Performance Metrics
- Number of participants
- Rate of participation growth
- Volume of quality data received
- Rate of data growth
- Valuable feedback from participants
These performance metrics help determine if your registry is off to the right start. Importantly, they are not an endpoint. Focusing on them alone will not guarantee you achieve your desired results.
For example, some registries have hundreds of sites participating and data for millions of patients or cases, but little to show in the way of results. They are focused on enrolling sites and getting data first, and the purpose value of the registry second.
You need to see the outputs — the results — of all of the inputs to evaluate your success.
Registry Success: Measurable Results
- Level of registry engagement (sessions, report views, dashboard interactions)
- Number of published research articles using registry data
- Rate of published research using registry data
- Development of evidence-based care guidelines
- Trackable improved outcomes from compliance with evidence-based guidelines
- Using registry data to shape healthcare policy and advance health equity
- Volume and types of data that can be repackaged and commercialized for industry
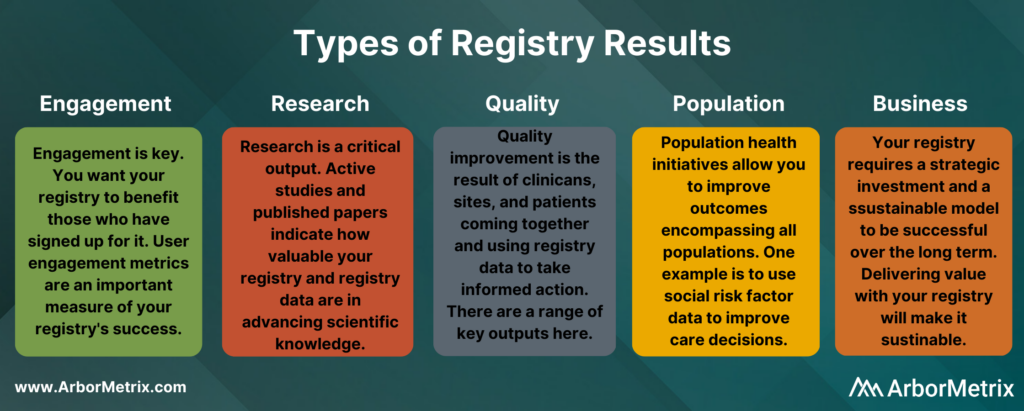
These results fall into five primary categories: Engagement, Research, Quality Improvement, Population Health, and Business Outcomes.
Engagement
User engagement metrics are a primary output of your registry. They signal to you whether those who have signed up for your registry are finding it useful.
You can use engagement metrics to understand how physicians and administrators are using your registry.
- Are they interacting with clinical outcome reports to identify best practices?
- In what ways are they drilling into reports to uncover trends and establish guidelines?
- When are they using predictive analytics and outcomes calculators for point-of-care and shared decision-making?
- How are they leveraging patient-reported outcomes (PROs) data?
When data demonstrate methods for improving patient care, physicians, researchers, and end users are easily engaged. They actively use the registry to define new and well-supported best practice guidelines and to support high-quality research.
Administrative dashboards are useful tools for tracking registry engagement by presenting live data across a variety of metrics – from report utilization to end user support requests. The dashboards provide critical information to identify best practices and drive further registry enhancements.
Patient Engagement
Engagement metrics do not only apply to physicians and administrators. They also apply to patients and family members who participate in patient-reported outcomes surveys and other patient engagement programs.
- What percentage of patients have responded to PRO surveys?
- How are patients and physicians using information to support shared decision-making?
- What resources are patients accessing through their engagement dashboard?
Research
Many registries leverage high-quality data and analytics to support research.
The results to measure here include:
- The frequency and volume of impactful publications using data-driven evidence from your registry.
- The speed to value your registry delivers in supporting active research. For example, ArborMetrix customers publish research in academic journals within 12 months of implementation go-live.
- The number of clinicians, researchers, or industry partners using your registry data as a foundation for registry-enhanced or registry-based clinical trials or post-market surveillance studies.
As soon as your registry becomes productive, spread the news. Disseminate findings early and often. When physicians, researchers, industry, and patients hear the latest, they will engage, participate, and contribute.
Here are a few ideas for sharing research among your community:
- Make a library of research publications using registry data available on your registry website and call out highlights on your home page. These are examples of research using data from our customers’ registries.
- Include registry-based research highlights in your member or community newsletter.
- Educate your member community and stakeholders about the findings of registry-based research during annual conferences, scientific meetings, and webinars.
- Share registry-based research publications on your social media profiles.
Quality Improvement
Typically, measurable quality improvement is achieved through a combination of the registry and collaborative efforts across participating sites and physicians. Many of our medical specialty society registry customers adopt the Learning Health System model to achieve lasting quality improvement.
Quality improvement results include:
- Development of evidence-based care guidelines.
- Adherence to evidence-based guidelines and best practices.
- Trackable (and often published) improved outcomes from compliance with evidence-based guidelines.
Population Health
Population health initiatives allow you to improve health outcomes across all populations. This includes using registry data to identify needs, act to address outcome gaps, and evaluate population health initiatives to inform best practices. There are many key outputs that indicate how well your registry is advancing population health.
Evidence of success with population health initiatives includes:
- Increase in engagement with initiatives aimed at addressing health disparities.
- Collaborative sharing of best practices to advance health equity.
- Increase in use of patient social risk factor data to improve care decisions.
- Growth of population health insights used to shape healthcare policy.
Business
The reality of running a registry is that it also affects your business operations. Whether you are a medical specialty society, patient foundation, or other healthcare association, your registry requires a strategic investment and a sustainable model to make it successful over the long term.
Business results for clinical and patient registries include:
- Amount of fees received from participating sites or physicians
- Amount of funding received from public and private grants
- Amount of revenue received from other sources, like industry partners who either sponsor part of your registry program or purchase data and reports
Successful clinical registries are designed with all of this in mind.
- How will your registry provide enough value and day-to-day utility to justify member or site fees, and/or how will you design a program that provides value to other industry stakeholders?
- How will your registry enable you to leverage partnerships that remove the financial burden for ongoing maintenance and innovation?
- What role does or could your registry play in supporting industry-driven research and development to track, document, and improve the safety and effectiveness of medical devices and therapies?
How Do I Achieve Success With My Registry?
There are a number of decisions—often made early on—that have a direct effect on the long-term success and sustainability of a registry. The technology you use to design and build the registry, your participation model, and data quality all affect your registry’s trajectory.
Before diving into it all, know this:
The single most important factor to registry success is knowing where you want to go. As the adage goes, “Begin with the end in mind.” Start there. Let your goals determine who participates, when and how you measure performance, and what data you use.
We take this approach with our customers by asking what you want to achieve and guiding you through a step-by-step process to design a registry with your goals in mind.
3 Technology Essentials for Clinical Registries that Improve Care

Improving care seems like a monumental undertaking. It sounds expensive. It sounds overwhelming. But the good news is, if you have a clinical data registry, improving healthcare delivery and advancing research has never been more achievable.
Here are three technology essentials to consider when designing, building, and managing a clinical registry that will get you moving closer to measuring and improving care.
Using Healthcare Analytics for Clinical Registries
Running a successful clinical registry, also called a patient registry or disease registry, is a significant undertaking that requires the right technology and the right approach. Today’s most valued and most effective registries use healthcare analytics that go beyond data hoarding and warehousing, and set the stage to play a crucial role in advancing care and research.
Specifically, registry technology should cover three essentials:
- Integrate: Efficiently and securely acquiring and validating healthcare data from various sources.
- Identify: Advanced analytics are used for building trust in the data and uncovering drivers of outcome and variation.
- Inform: Delivering insights via interactive reports and tools that inspire data-driven action.
3 Keys of Registry Technology
1. Integrate: Collecting and Validating Data with Clinical Registry Software
Successful data acquisition creates a complete (and sometimes large) data set. Key aspects of this step include data sources, data integration technologies, and validating data for quality.
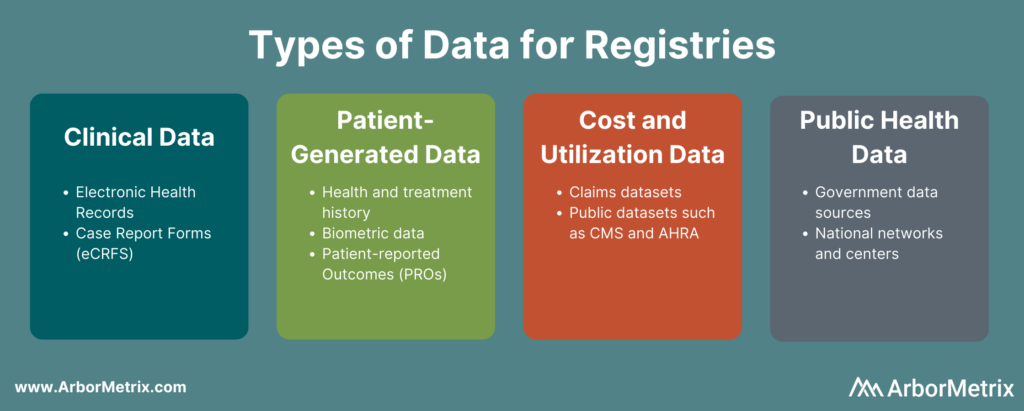
Registry Data Sources
Registries that achieve measurable results rely on a diverse and complete data set. They harmonize data from many sources, including:
- Clinical data from electronic health records (EHRs) and case report forms (eCRFs).
- Patient-generated data from patient-reported outcome (PRO) surveys.
- Cost and utilization data from claims and public datasets.
- Public health data from various government data sources.
Data Integration Technologies
In order to drive participation and value, registries must use efficient electronic data collection methods that ingest, validate, and transform data into a high-quality asset.
- Ingest: Deploy standards-based, vendor-specific, and custom connectors to ensure interoperability across EHR and other systems and sources.
- Validate: Ensure data completeness, correctness, and accuracy.
- Transform: Assemble and organize data into a single consistent representation.
Importantly, successful registries should base their data acquisition strategy on their goals and purpose – not just the data most readily or easily available to them. Focusing on what you need rather than on what you can get will point your registry in the most successful direction.
2. Identify: Analyzing Healthcare Data to Uncover Opportunities
Applying advanced analytics ensures data and reports are credible and trusted. When it comes to building engagement in trusted data, analytics make the difference. Here are a few specific methodological and statistical tools your registry should use.
Risk Adjustment
Risk adjustment is a process that corrects for the severity of a patient’s illness. Adjusting for risk levels the playing field and ensures that comparisons of hospitals and clinicians are fair and accurate. For example, our registries use scientifically and clinically validated statistical risk models to evaluate – in real-time – all factors related to each outcome. This builds trust.
Reliability Adjustment
In healthcare quality measurement, when sample sizes for a hospital or clinician are small, the observed rates or rare outcomes may be due to chance and should be considered less precise than rates based on larger sample sizes. Reliability adjustment is a statistical technique that is designed to isolate the signal and reduce the noise in your dataset. Specifically, our methods will determine whether outcomes are due to chance or true differences in quality.
If you want to learn more about risk and reliability adjustment, check out this post: What Are Risk and Reliability Adjustment and Why Do They Matter?
Benchmarking
Healthcare benchmarking means comparing a hospital or practice, or clinician, to others. Your registry should provide benchmarking based on patient characteristics, condition, geography, facility type, or other meaningful groups. It’s smart to also blind these benchmarks to protect privacy while still supporting effective measurement.
Peer Group Definition
Peer groups are an important part of benchmarking. Look for reporting and peer benchmarking capabilities at the clinician, practice, multi-practice, multi-facility, and organization levels.
If you want to learn more about benchmarking in healthcare, check out this post: What Is Healthcare Benchmarking?

3. Inform: Delivering Insights and Leveraging Evidence
While leveraging analytics and data science unleashes the value of your registry data, making those insights and reports accessible, engaging, and easily understood unlocks exponential value for your organization.
To make those insights as valuable as possible, aim to prioritize the user experience. You’ll want to think of your registry participants and end users as people who have a wide exposure to a variety of digital experiences. That means that when it comes to engaging with your registry, participants:
- Expect the same technological experience as their favorite apps or websites.
- Share and/or use information when it is convenient and compelling.
- Need their experience to be valuable.
When you focus on user experience, it ensures that every person can get value from the data, whether their needs are simple or complex. We’ve designed reporting features in our registry platform to drive engagement and support data-driven decision making. A few of the specific reporting features we use in our registry platform to drive patient engagement and support analysis include:
- Intuitive Views: Analyze data at the patient, provider, or site level, either individually or in aggregate.
- Flexible Interaction: Explore data at a deep clinical level by creating custom patient cohorts, analyzing case-level details, and drawing correlations within and across diagnoses.
- Personalized Visualizations: Perform complex analyses and create powerful visualizations using embedded Tableau.
- Custom Queries: Extract raw or analyzed data for special research projects or reports.
If you want to learn more about our user-centered approach to healthcare analytics software design, check out this post: Designing Healthcare Analytics to Engage Clinicians.
Registry Analytics Checklist
How do you use analytics to ensure measurement is fair, trusted, and engaging?
Download this checklist to help you evaluate your registry analytics.
Other Interactive Experiences for Registries
Beyond reporting and data visualizations, registries can harness emerging tools and technologies to add utility and value to their programs.
Patient Engagement and Patient-Reported Outcomes
Patient data informs a more complete view of quality, smoothing the gaps between clinical visits documented in the EHR, and helps you achieve even greater results. Modern patient-reported outcomes surveys and engagement tools allow you to collect the right data from the right patient at the right time.
Clinical Decision Support
Bringing together clinical data and patient-reported outcomes creates a powerful asset. Outcomes calculators use predictive analytics to intelligently combine registry data to predict outcomes, risks, and results of procedures, therapies, and treatments. This enables physicians to make data-driven decisions and empowers patients to engage in informed conversations about their outcomes.
Putting It All Together
These three essential steps to a successful registry are complex but achievable. ArborMetrix has built strong partnerships with our clients that put acquiring, organizing, analyzing, and acting on data at the center of their registry strategy.
Our clinical registry platform and our strategy and partnership, put these goals in their grasp. We bring our proven strengths in delivering clinical data registries that lead to scientific findings and actionable insights that have a real impact on real people.
The Basics of Clinical Data Registries

Healthcare’s digital transformation is long underway, yet still lagging. Calls for better and more efficient care, therapies, and outcomes have never been louder and more insistent. The role of clinical data registries has never been more significant.
It’s necessary to understand the importance of registries and how they make sense out of large volumes of disparate healthcare data to measurably improve care and advance research.
Let’s cover the basics:
- What Is a Clinical Data Registry?
- Types of Clinical Registries
- Purpose and Uses of Clinical Data Registries
- The Value of Clinical Data Registries
- Examples of Quality Improvement with Clinical Data Registries
- Example of a Medical Device Registry
- Getting Started with Clinical Data Registry Software Solutions
What Is a Clinical Data Registry?
A clinical data registry is an interactive database that collects, organizes, and displays healthcare information.
Clinical data registries are also sometimes called patient registries and disease registries. Professional medical associations and specialty societies tend to use the term clinical data registry, while research and patient foundations and government organizations lean toward patient registry. Because disease registries sound condition-specific, that term is often more popular with industry.
Regardless of the name, the purpose of a data registry is the same: to evaluate and improve outcomes for a population defined by a particular condition, disease, or exposure.
Specifically, registries use observational study methods to collect and harmonize data about the treatment, outcomes, and well-being of patients who receive care over time. They aggregate large data sets and analyze trends or patterns in treatments and outcomes.
Registries can serve many purposes and provide value for a variety of healthcare stakeholders. For example:
- Physicians and other healthcare professionals use registries to evaluate available treatments, procedures, and therapies, and to understand how patients with different characteristics respond to various treatments.
- Medical device manufacturers and pharmaceutical developers use registries to track and understand the effectiveness, safety, and value of medical devices or therapies and drugs entering or on the market.
The number of registries has grown over the past several decades as healthcare information has become digitized. Yet despite their increase in use and significance, registries face real challenges in establishing the participation, engagement, and utility needed to drive their sustainability.
Modern clinical data registries address these limitations by going beyond data collection and data warehousing. They rely on advanced analytics and data science to transform data into meaningful insights that are useful, usable, and used by a variety of stakeholders to achieve a desired outcome.
Types of Clinical Registries
Clinical registries come in many different forms. The type of registry depends on the organization managing or sponsoring it, and the patient population, disease, condition, or treatment it examines.
Although registry goals and purposes vary, when designed with the right approach and built with the right analytics technology, they can measurably improve care.
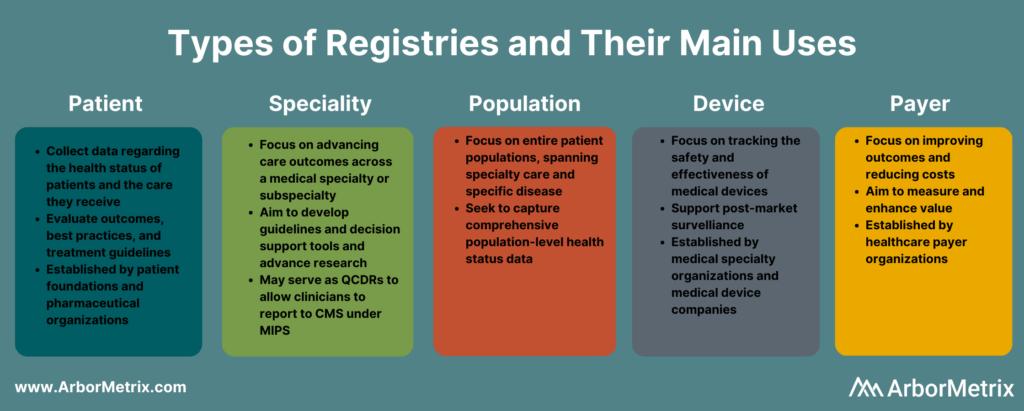
What Is a Patient Registry?
A patient registry, also called a disease registry, tracks information about the health status of patients and the care they receive for a specific disease or condition. Patient registries bring together data to evaluate longitudinal outcomes, best practices, treatment guidelines, and to support research and therapeutic development.
A growing number of patient foundations and pharmaceutical organizations are establishing patient registries to study the treatment of rare diseases and conditions, such as hemophilia and other genetic diseases.
What Is a Specialty Registry?
Specialty registries are clinical registries focused on advancing care and outcomes across a medical specialty or subspecialty, such as pathology, sleep medicine, surgery, and trauma medicine. These registries often aim to develop guidelines and decision support tools, accelerate research, and advance care through collaborative quality improvement.
What Is a Population Registry?
A population registry is more broadly focused across entire patient populations and spans both specialty care and specific diseases and conditions. These registries aim to capture the health, well-being, diagnostic, treatment, and outcome data for every patient within a population defined by demographics (age, gender, or other social determinants), geography (state, region, country and including like Health Information Exchanges and within Health Departments), or disease or condition (diabetes, cancer).
What Is a Medical Device Registry?
A medical device registry is focused on tracking the effectiveness, safety, and value of medical devices. Device registries come in several forms. Medical specialty organizations may collect data on various devices used for procedures or conditions, as part of their clinical data registries. Medical device companies establish registries and use registry data to support post-market surveillance.
What Is a Payer Registry?
A payer registry is established by a healthcare payer focused on measuring and improving value by advancing outcomes and reducing costs. Payer-sponsored registries are often organized across a specific geography or region, and by specialty – surgery, urology, emergency medicine, etc.
Purpose and Uses of Clinical Data Registries
Healthcare organizations such as medical specialty societies, patient foundations, pharmaceutical companies, and medical device manufacturers establish registries for many purposes and uses.
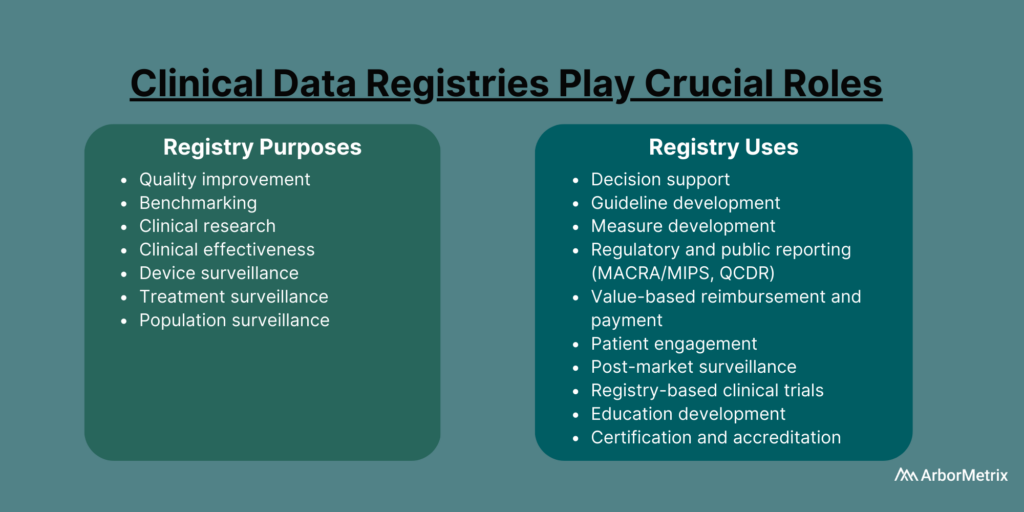
Clinical Data Registry Purposes
- Quality improvement
- Benchmarking
- Clinical research
- Clinical effectiveness
- Cost effectiveness
- Device surveillance
- Treatment surveillance
- Population surveillance
Clinical Data Registry Uses
- Decision support
- Guideline development
- Measure development
- Regulatory and public reporting
- Value-based reimbursement and payment
- Patient engagement
- Post-market surveillance
- Registry-based clinical trials
- Education development
- Certification and accreditation
The Value of Clinical Data Registries
Clinical data registries are valuable when they measurably improve care and achieve results. Examples of this in action are advancing research, establishing and evaluating guidelines, or managing and reducing costs.
Achieving value with a registry happens when:
- Physicians and providers use high-quality, data-driven insights to better understand expected outcomes, make evidence-based decisions, and share best practices.
- Patients share timely and personal data about their condition and outcomes and gain a greater understanding of their care that leads to informed shared decision-making.
- Researchers and developers use registry data as the foundation for registry-enhanced or registry-based research, clinical trials, or post-market surveillance studies.
Examples of Quality Improvement with Clinical Data Registries
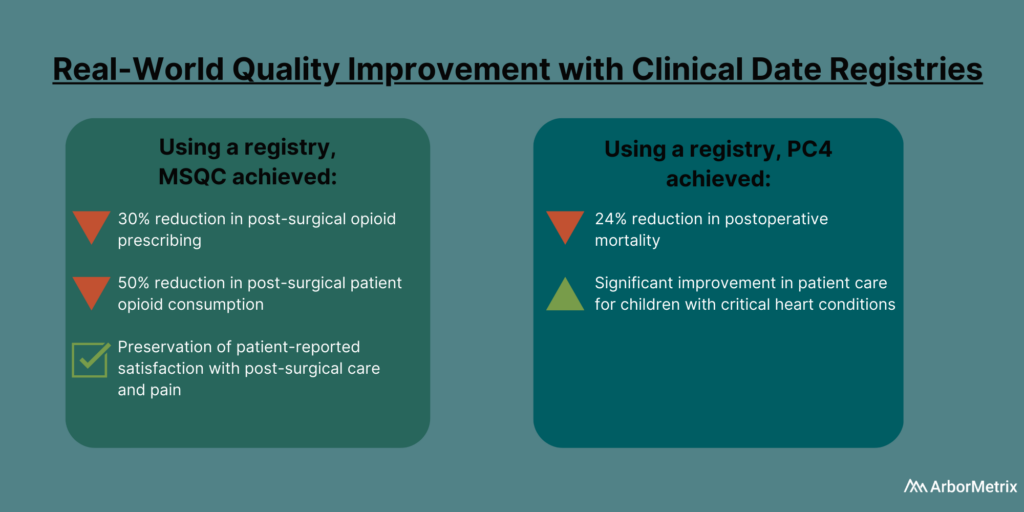
In 2019, the Michigan Surgical Quality Collaborative (MSQC) used registry data to generate knowledge in the form of procedure‐specific opioid prescribing guidelines.
The result?
Post-surgical opioid prescribing dropped by 30%, and post-surgical patient opioid consumption dropped by 50%, according to a paper published in the New England Journal of Medicine. There also was no change in patient-reported satisfaction with care and pain in the week after surgery.
The Pediatric Cardiac Critical Care Consortium (PC4) aims to improve the quality of care for pediatric heart patients through a clinical data registry that allows hospitals to evaluate their own outcomes and learn best practices.
Their efforts are paying off.
Eighteen hospitals significantly reduced mortality and improved care for children with critical heart conditions, according to a paper published in the December 2019 edition of the Journal of the American College of Cardiology. Specifically, they achieved a 24% decrease in postoperative mortality among participating sites between 2014 and 2018.
If you want to know more, read this post about how MSQC and PC4 are top examples of quality improvement in healthcare.
Getting Started with Clinical Data Registry Software Solutions
Today’s most successful clinical data registries use healthcare analytics technology that goes beyond data collection and data warehousing and plays a crucial role in advancing care and research.
Specifically, a clinical data registry platform should:
- Acquire various data using industry-leading technology and standards.
- Assemble the data into real-world evidence using advanced analytics and data science.
- Enable various users to act on the evidence using dashboards, reports, surveys, and other unique decision-support tools.
Leverage the Power of Clinical Data Registries
The National Quality Registry Network outlines some key considerations when approaching a clinical data registry and deciding what to outsource to a vendor. This includes your in-house availability and expertise, budget impact, convenience, and the many responsibilities that can be outsourced or kept in-house.
At ArborMetrix, we help healthcare organizations and companies demonstrate real and measurable results through robust analytics and intuitive reporting. Through our comprehensive partnerships and clinical expertise, we enable our clients to leverage their real-world evidence for real-world results.