How Registries Gain Momentum and Achieve Lasting Value

Here at ArborMetrix, we’re focused on creating and delivering powerful product roadmaps that align with the industry and the needs of our clients and partners. These roadmaps help prioritize work, set expectations, and point us ever forward, toward our long-term vision.
But, roadmaps aren’t simply our own internal tools. We often provide roadmaps to our customers for their clinical registries. These plans outline how to take their data and software assets to the next levels for their populations. To be effective long term, your registry must continually evolve to address the growing needs of your clinician and patient populations.
Clinical registries exist on a scale of momentum that spans three stages:
- Foundational
- Intermediate
- Advanced
We guide our registry partners through each stage and set a strategic plan to help them progress to their ultimate goals.
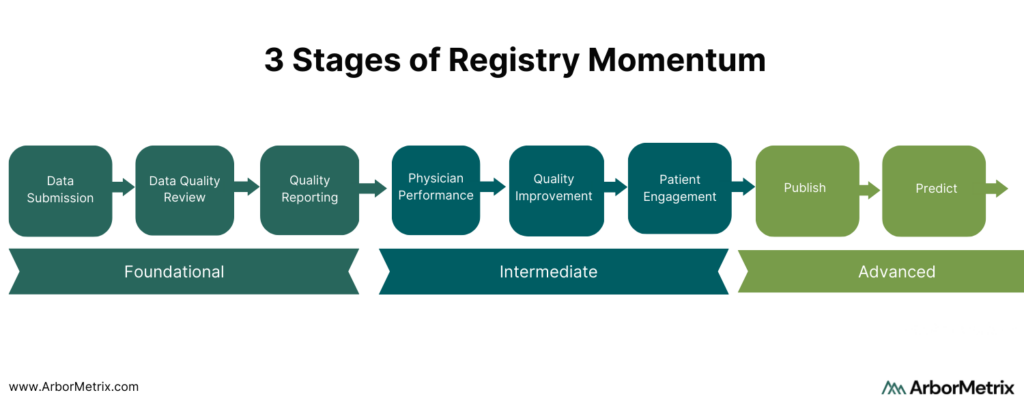 Foundational Registry Momentum: Data Submission, Data Quality Review, Standard Reporting
Foundational Registry Momentum: Data Submission, Data Quality Review, Standard Reporting
Foundational registry momentum is defined by the compelling business and market needs that drive a registry’s framework. Often the impetus is collecting data for regulatory or post-market reporting, with features that include:
- Collecting data through a variety of channels and formats.
- Ensuring data is high quality through a robust data dictionary and supporting validation logic.
The Foundation stage is important because it serves to get data in the registry and drives membership and participation from affiliated organizations and clinicians. Most new registries achieve these goals within the first six months of launch.
However, if your registry launched a few years ago and you are feeling like your momentum is “stuck” or “limited” by not progressing past the Foundational stage, you’re not alone. This is a common situation that you can remedy with the right approach and technology.
Intermediate Registry Momentum: Clinician Performance, Quality Improvement, Patient Engagement
The momentum of your registry is truly unleashed when it builds deeper engagement.
This begins when it is used to highlight and change behavior. Our registry product and services are purpose-built for deep analytics and actionable insights related to measurable clinician and device performance, quality improvement, and patient engagement.
- Clinicians and industry stakeholders engage when the registry can be used to highlight their performance relative to peers and within their health system or specialty.
- Quality improvement is realized when the registry can indicate areas where treatments and interventions can be improved, and where guideline adherence is tracked and analyzed for impact.
- Patients engage when they are given the opportunity to safely share their health and quality of life related to diagnoses, treatments, and interventions, and know that data can be used in shared decision making.
Most of our clients spend a significant portion of their registry’s lifecycle building and growing these intermediate assets. This starts with our goals-oriented implementation process. These features can propel the momentum into new and relevant measurement areas and reporting domains that keep a registry fresh and engaging over time.
Advanced Registry Momentum: Publish and Predict
Finally, let’s talk about the hallmark of registry success: The ability to use it as a platform to publish research and clinical practice guidelines, and to predict outcomes. Advanced registries also expand their scope of participation and achieve sustainability through data commercialization.
Here’s how they reach this level. Registries arm us with trusted insights on clinician and device performance and quality improvement in care delivery. They also inform the patient experience through patient-reported outcomes. Registry owners and stewards share this knowledge and insights with the broader community to extend its impact.
Clinical Research and Practice Guidelines
Our customers have published a rich portfolio of registry-based research. Importantly, we help them achieve rapid-cycle research. On average, our customers publish their first peer-reviewed publication using registry data within a year of launch.
This is not just within scientific and medical journals, but also with the creation and publication of new clinical practice guidelines. The rigorous scientific process that we apply to registry data makes it ideal for evaluating the standards of care and long-term outcomes that impact patients.
Predictive Analytics and Outcomes Calculators
Predictive analytics is when data science principles are applied to model and forecast outcomes and risks at the point of care. This allows clinicals to make treatment decisions with better insight and, ultimately, avoid complications and negative outcomes.
The most advanced registries we support utilize outcomes calculators. These are built on complex data and models that combine foundational data assets with longitudinal patient data. Outcomes calculators provide powerful tools for shared decision-making between providers and patients and provide long-term registry value.
Predictive Analytics Registry Example
One example of an advanced registry using predictive calculators is the Michigan Bariatric Surgery Collaborative (MBSC). MBSC aims to advance the science and practice of bariatric surgery. Registry data is used to feed the MBSC Predictive Outcomes Calculator, which is publicly available for clinicians to use to predict a patient’s weight loss, comorbidity resolution, and complication rate after bariatric surgery.
Specifically, the tool:
- Predicts weight loss at years 1, 2, and 3 for five different procedures using patient information on demographics, comorbidities, and risk factors.
- Predicts the likelihood of resolving weight-related comorbidities.
- Predicts the likelihood of minor and major complications.
Notably, the predicted rates for weight loss, comorbidity resolution and potential complications are patient-specific, using risk-adjusted, real-world outcome data from similar patients.
These tools and other quality improvement initiatives helped MBSC and its members decrease rates of VTE by 43% and decrease post-surgical death rates by 67%.
Achieve Lasting Value with Your Registry
It’s important to remember that registry momentum happens over varying spans of time.
Some registries have been around for many years, and their momentum has been focused on providing foundational value to its members. Other registries are only getting started and are already looking at how they can propel their programs by quickly predicting outcomes and publishing results.
Whatever stage in which your registry exists, we have both the vision and the delivery model to help you build momentum and achieve lasting value.
The Top 3 Lessons for Registries from the CMSS Annual Meeting

The Council of Medical Specialty Societies (CMSS) held their 2020 Virtual Annual Meeting with the theme of Covid-19 and Beyond: Digital Transformation of Healthcare, Research, and Education.
The dynamic group of presenters and panelists covered a variety of topics that demonstrate how digital technology, such as clinical registries and healthcare analytics, can be instrumental in advancing healthcare.
Our key takeaway: The digital transformations that are needed to make these advancements are less about how people transform technology and more about how technology transforms people and lives.
Registries, for instance, can be used to help solve some of the most intractable issues in healthcare like:
- Addressing underlying root causes of health disparities.
- Making it easier for patients to understand, navigate, and manage their care.
- Monitoring and managing COVID-19 and other emerging priorities.
1. Addressing Root Causes of Health Disparities
There are widespread and longstanding health disparities among racial and ethnic minorities.
These disparities cause some populations to face unfair health burdens. Health is a fundamental human right, and consequently, healthcare stakeholders have an ethical obligation to ensure fair and just health experiences by acting to eliminate avoidable health disparities.
Clinical data registries are powerful tools for assessing health disparities, identifying the root causes, and tracking efforts to address health inequities. Importantly, these tools should provide actionable insight, as referenced by Rhea Boyd, M.D., M.P.H., Pediatrician and Child Health Advocate at Palo Alto Medical Foundation and UCSF Benioff Children’s Hospital.
This work includes implementing robust data logic rules to identify underperformance in need of equity-focused quality improvement efforts, as presented by Ernest Moy, M.D., M.P.H., Executive Director, Office of Health Equity, U.S. Department of Veterans Affairs. Those health equity efforts are made more effective with social determinants of health analytics that provide a comprehensive view of the interconnected factors influencing a patient’s health.
How Registries Can Advance Health Equity
Alongside several of our partners at medical specialty societies, we see the very real effects of longstanding and systemic health disparities – made evident through evaluating data in their registries.
Registries collect a high-volume of data from different sources and deliver high-quality, data-driven insights. Efforts to reduce health disparities require data to detect and measure the impact of disparities, identify the complex and interconnected determinants, and inform interventions and best practices.
Efforts to advance health equity through registries fall into three activities:
- Measuring health disparities. To reduce health inequities, we need methods to measure disparate health outcomes and methods to assess the impact of those disparities across different types of health indicators.
- Assessing underlying factors. Effectively addressing health disparities requires understanding the underlying factors influencing disparate outcomes.
- Forming and evaluating interventions. By assessing health disparities and their underlying factors, we will have insights to develop impactful interventions and sustaining widespread health equity improvements through tracking best practices.
2. Helping Patients Understand Their Care and Outcomes
Healthcare is complex. Patients can be overwhelmed as they try to understand, navigate, and make important decisions about their care.
Consider a patient with a chronic condition who might have up to 20 healthcare providers, all generating assessments and treatment plans that must be coordinated and aligned. Even people who have spent their life working in healthcare can feel overburdened by the amount and complexity of information presented to patients.
More patients are now turning to technology to help them through their care journey. And now more technology is becoming patient-centric to help patients overcome common barriers they face in their healthcare.
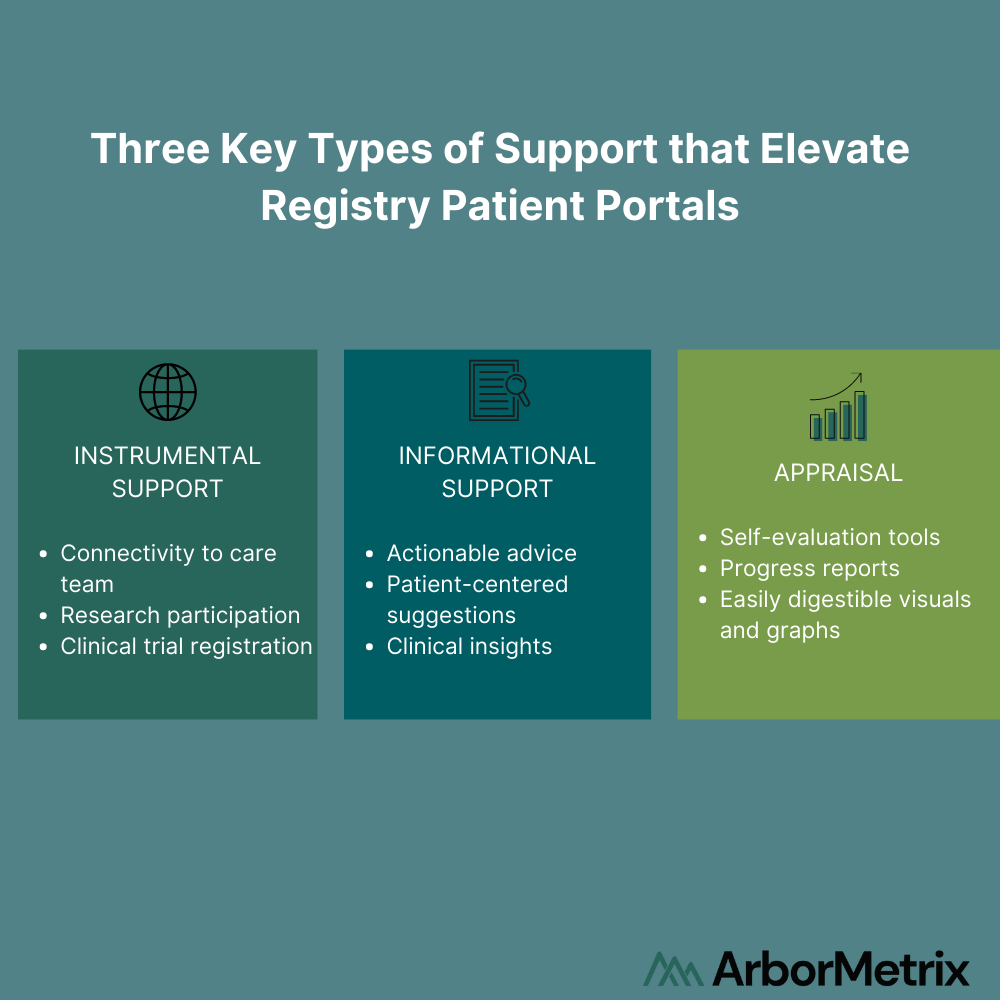
For example, registries can offer patient engagement tools that provide patients with meaningful support as they interact with their health. Registry patient portals should offer key types of support to improve patient experiences and health outcomes:
- Instrumental Support. Tangible services and functions, such as capabilities to directly connect to their care team or participate in clinical trials or research.
- Informational Support. Connection to tailored patient resources that provide advice, suggestions, and clinical insights.
- Appraisal. Personalized registry data can be presented back to patients in easy-to-review graphs. This can show patients how they are progressing throughout their treatment, which helps patients to self-evaluate their progress and health experiences.
As described by Donna Cryer, JD, President and CEO of the Global Liver Institute, these patient-centric technologies give patients supportive communities and influential tools to elevate a patient’s voice and help them maximize their health.
3. Understanding a Global Pandemic
Monitoring, managing, and understanding COVID-19 was top-of-mind throughout several sessions. Registries are playing a central role in this.
Modern registries can address emerging health issues like the pandemic with rapid measurement and quick discoveries.
There are three key parts to doing this successfully:
- Determining the priorities and research questions a registry can uniquely answer.
- Using trusted data and advanced analytics to address critical issues.
- Leveraging a flexible data infrastructure to pivot at any time – modern clinical data registries are designed to evolve.
Several organizations are already leveraging their registries and data infrastructure to be proactive in measuring, monitoring, and addressing the COVID-19 pandemic. You can learn from them and find more strategies in our blog post “Best Practices for Clinical Registries with New Research Priorities.”
Registry Technology Should be People-Centric
Overall, digital transformations are about using technology to solve issues for people, not simply about using people to solve technology issues.
It is less about being technology-centric than it is about making technology people-centric. Registries are powerful tools that can be designed to address some of the most widespread health issues affecting people, while also engaging patients in innovative ways to improve their health experiences.
From defining the vision for a registry program to designing surveys and reports for clinicians and patients, we take a person-driven approach.
4 Ways Clinical Registries Deliver Value to Life Science Companies
 Medical specialty societies were founded on two key principles: knowledge sharing and the exchange of clinical insights to improve medical practice. That central task has evolved as societies use clinical data registries to serve diverse and important stakeholders who are also involved in ensuring optimal health outcomes.
Medical specialty societies were founded on two key principles: knowledge sharing and the exchange of clinical insights to improve medical practice. That central task has evolved as societies use clinical data registries to serve diverse and important stakeholders who are also involved in ensuring optimal health outcomes.
These clinical data registry stakeholders include:
- Clinicians
- Health Systems, Hospitals, and Provider Organizations
- Patients
- Public and Private Payers, and
- Life Science Organizations (Pharmaceutical and medical device companies)
Societies and their registries are vehicles to further data-driven care through collaboration with these health care stakeholders. New opportunities frequently arise for registries to add unique value through the data insights they provide — especially through partnerships with life science organizations.
Specifically, there are four common ways for registries to empower life science organizations with new understandings in patient outcomes. These opportunities further support real-time insights in clinical care.
- Patient Cohort Analytics
- Post-Market Surveillance
- Clinical Decision Support
- Registry-Based Clinical Trials
Let’s examine each to understand their value to industry and value to your mission.
1. Patient Cohort Analytics and Registries
Patient cohort analytics are useful in several ways. They provide analytic tools to facilitate activities like clinical trial enrollment and registry cohort comparison for research.
Registries transform raw data into tools that provide tremendous value to trial sponsors in two ways:
- Identification of individual patients who match a specific cohort of interest and meet clinical trial inclusion and exclusion criteria. This allows trial sponsors to expand their trial network beyond standard academic centers and into community care settings, and through rapid enrollment of trial participants.
- Creation of comparison cohorts of registry patients through propensity-score matching. This allows trial sponsors to allow for fair and accurate comparison between treatment cohorts within a trial external to the registry and non-treatment patients found within the registry.
2. Post-Market Surveillance and Registries
Patient registries are uniquely useful in their ability to facilitate post-market surveillance for three reasons:
- They advance post-market surveillance activities to help us better understand the real-world safety and effectiveness of procedures, treatments, and devices.
- They solve complex problems for device and pharma companies. These organizations in general are faced with complex barriers when seeking real-world data for purposes of research and development, quality improvement, or regulatory requirements.
- They deliver the comprehensive technology needed to build a registry that collects real-world data, transform it into real-world evidence, and make it accessible and useful.
Simply stated, registries provide real-time answers to common post-market surveillance questions, like:
- How is a device or drug actually used in real-world settings?
- What does the average patient look like?
- How does a product truly perform?
3. Clinical Decision Support and Registries
Clinical decision support tools convert retrospective registry data into powerful point-of-care tools with immediate impact on patient care.
Decision support tools are built to take the guess work out of trade-off decisions between treatment courses. With real-world data and a user-friendly interface, decision support apps are valuable resources. They enable clinicians and patients to prioritize outcomes and establish appropriate expectations.
At the same time, decision support tools can be designed to demonstrate the degree to which device or pharmaceutical factors drive outcomes. These tools create significant value for industry stakeholders and set the stage for value-based reimbursement.
4. Clinical Trials and Registries
Registry-based clinical trials (RBCTs) set the gold standard for creating value for multiple stakeholders.
RBCTs rely on the registry’s network of participating clinicians, their patients, and the foundational registry dataset. They identify participating investigators and clinicians, facilitate patient enrollment, and support the majority of data elements required for trial completion. Registries also capture specialized clinical trial data through custom patient surveys, electronic case report forms, and data mapping.
The process ultimately leads to a clinical trial completion that is much quicker and more cost-effective than traditional methods.
 Registry Data Partnership Readiness
Registry Data Partnership Readiness
There are a few key steps to determining your organization’s readiness for registry data partnerships with industry.
- Understand the market opportunity. To design an effective program, conduct market research to understand who would find value in your registry data, what products or therapies they have on the market, and how those products are valued.
- Assess your registry data. You’ll need to know what data in your registry would be valuable to medical device manufacturers or pharmaceutical companies. Assess those clinical situations where real-world registry data tracks either clinical practice or patient populations that can’t be easily studied via traditional trials.
- Validate your registry data quality. Data quality is important for any registry work. Before initiating registry-based clinical trials or making de-identified registry data available to industry, you want to ensure the data quality is regulatory-grade. Achieving this requires a thorough data quality analysis to assess data integrity across dimensions such as completeness, concordance, precision, and currency.
Developing Registries for Many Stakeholders
Registries are powerful tools for effectively generating new clinical insights for key stakeholders and creating meaningful partnerships with life science companies.
These stakeholder collaborations use registries to employ valuable data tools to evaluate and improve patient outcomes, and in the process, help to further support the operations of registries and their underlying missions.
ArborMetrix offers an industry-leading, proven registry solution that meets the needs of medical device and pharma companies, medical specialty societies, and clinicians.
Clinical Registries Are the Next Step for National Healthcare Improvement Initiatives
 In the alphabet soup of healthcare, often the initial drivers of large-scale initiatives are government reporting and regulatory requirements, like All Payer Claims Databases (APCD) and Health Information Exchanges (HIE). These requirements encourage interoperability and data sharing between stakeholders.
In the alphabet soup of healthcare, often the initial drivers of large-scale initiatives are government reporting and regulatory requirements, like All Payer Claims Databases (APCD) and Health Information Exchanges (HIE). These requirements encourage interoperability and data sharing between stakeholders.
These efforts aren’t new or news – but the past decade has focused on identifying assets, bringing the data in, and analytics that match patients across sources.
Government agencies at all levels (both in the U.S. and abroad) are moving into the next phase of this journey. They are going beyond data access and blending and focusing on using the data strategically to improve care and lower costs.
Registries Power Population Health Programs
When faced with a large population data set, it can be a challenge to target a single focus area that has both meaning and impact. Clinical data registries are a perfect example of macro-level data used for micro-level initiatives. When these targeted initiatives achieve measurable results, this leads to tremendous power for population health initiatives.
We’ve seen the role registries play in quality improvement successes at specialty-focused organizations. They’ve driven important and renowned innovations in care delivery. This has been accomplished through the symbiotic relationship between a culture of engaged physicians and the use of trusted data and powerful technology. The technology allows for a window into physician performance and patient outcomes.
When planning how to make a patient registry part of your population health program, there are two parts to consider:
- Registry Technology
- Registry Strategy
Part 1: Technology of Building a Clinical Data Registry for Population Health
A clinical data registry is built on the ingestion of real-world data and the transformation of that data through advanced analytics into actionable real-world evidence. Let’s break this process down into 3 key steps.
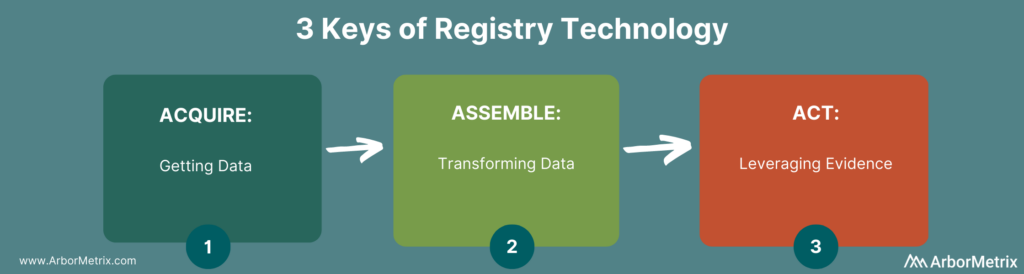
- Data Acquisition: Acquisition can include a broad spectrum of options. One option is machine-to-machine connections to EHRs, government assets, and other databases. There are also human-to-machine connections as needed for data entry from providers and patients. This includes more granular disease-specific elements and longitudinal outcomes outside of routine care visits.
- Data Assembly: Assembly can include validation and cleansing for quality, and transformation for analytic purposes. This includes creation of clinically specific measures, risk adjustment for fair case mix comparisons, patient matching, benchmark creation, predictive models, and more. This transformation is key to building trust in the data.
- Acting on Evidence: Leveraging evidence is facilitated by our intuitive and user-friendly AMxCore product that provides visibility into all individual data elements attributed to patients, as well as aggregate reporting that provides deep insights into trends, patterns, and opportunities for change.
In large initiatives, so much time is spent on acquiring data that transforming it and utilizing it effectively are afterthoughts. While data is foundational and fundamental, a strong strategy behind the technology ensures a speed to value and long-term registry success.
Part 2: Strategy of Building a Clinical Data Registry for Population Health
A registry toolset needs to go beyond technology and data access and produce information of such quality that it can be relied upon to improve the standard of care across entire health systems, states, or countries.
At its strategic core, a registry is a very simple concept broken into 3 parts.
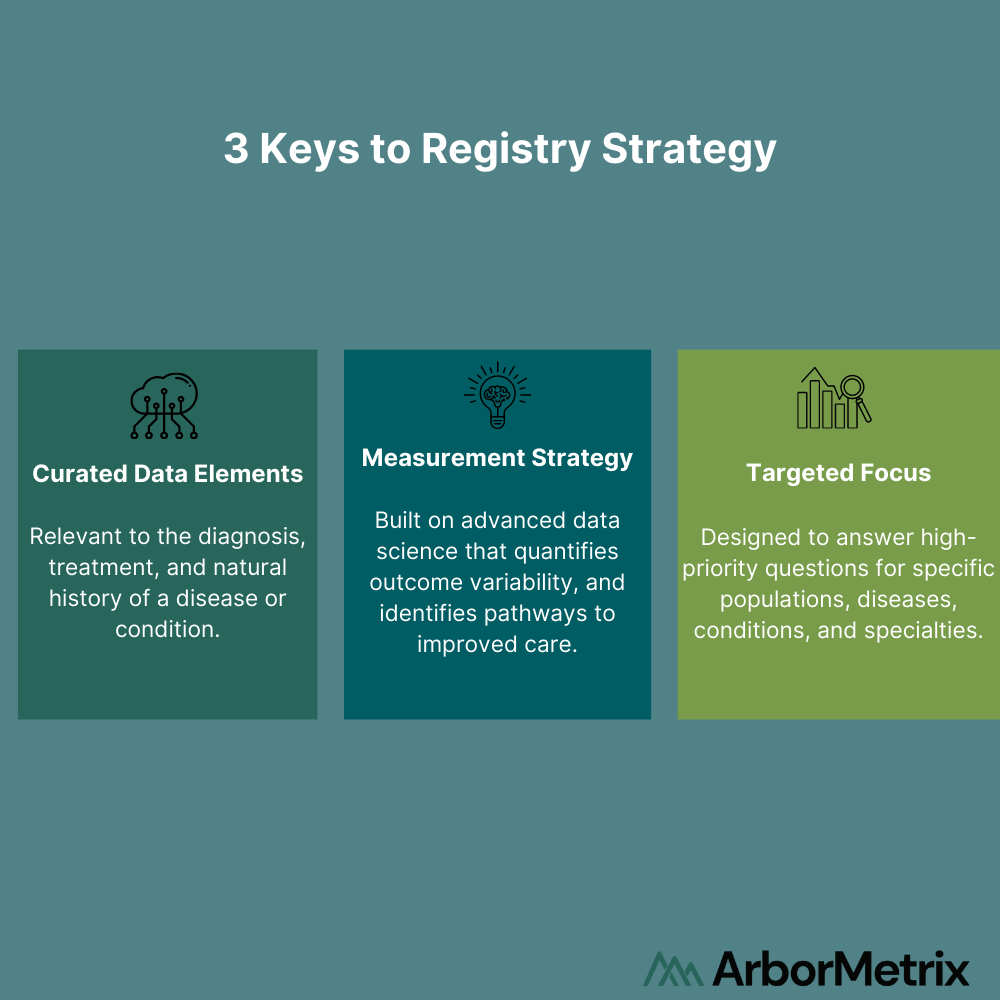 Curated Data Elements
Curated Data Elements
The curated data elements are considered and selected from the broad set of data assets available and used to create a defined Data Dictionary. While in some cases, gathering as many data elements as available has value (often the foundation of initiatives like HIEs and APCDs), most successful quality improvement initiatives only utilize a subset of these elements. Strategically curating these assets ensures the most relevant and meaningful elements are being collected, validated, and transformed for purpose-driven use in the context of specific diseases or conditions.
Measurement Strategy
The measurement strategy transforms data into information through the rigorous application of data science principles. These principles create trusted data assets that can quantify outcomes and identify the drivers of those outcomes. This trusted data gives, agencies, hospitals, or physicians the resources they need to better understand and improve care. This is the strategic heart of a registry – data that is trusted to enact important care delivery changes.
Targeted Focus
A targeted focus is the identification of high-priority questions your agency or organization needs to answer about the providers, patients, and processes under your influence. Starting with a focus ensures that you are pointed at achievable opportunities for change.
Think: reduce surgical site infection rates, not reduce mortality. This focus ensures that you are targeting true opportunity for improvement that will be measurable and repeatable across the system of care.
Operationalize Large Scale Quality Improvement
When you apply this strategic framework on top of strong registry technology, you can operationalize and catalyze large scale improvements in care delivery – across organizations, agencies, and around the world. To learn more about how to evaluate your registry technology, check out my post: 3 Technology Essentials for Patient Registries that Improve Care.
Best Practices for Clinical Registries with New Research Priorities

You set goals, outlined plans, and hit milestones. Then 2020 changed - in a big way. Questions came. Priorities shifted. Opportunities emerged.
Despite uncertainty, there are a few things to be sure about:
- Registries are essential to our understanding of the direct and indirect effects of the pandemic.
- Registries must adapt to address emerging research priorities and questions.
- Registries can pivot successfully with the right strategy, technology, and partners.
Successful patient registries are designed to pivot. Flexibility is inherent and technology scales as needs evolve. Consider how the following organizations changed course to address pressing issues and emerging research questions.
- The current healthcare landscape: The American Academy of Physical Medicine and Rehabilitation (AAPM&R) is adapting its registry to answer important research questions and track long-term patient outcomes.
- The COVID-19 pandemic: The Acute Care Quality Registry quickly adapted its technology to monitor and manage the effects of the pandemic on patients, hospitalists, and health systems.
- The national opioid epidemic: The Michigan Surgical Quality Collaborative leveraged its registry to establish evidence-based guidelines, track adherence to those guidelines, and ultimately reduce post-op opioid prescribing by 30% and consumption by 50%.
Step 1: Determine the Priorities and Research Questions Your Registry Can Uniquely Answer
Often with a registry, it's tempting to start with the data you can easily collect and then make sense of it all. But this quickly leads to overwhelming data volume with a lack of specificity.
Instead, flip the script and begin with the end in mind. Use this framework and ask yourself the following:
- What are your goals and objectives? How have they changed? Which ones are inherently important – no matter what changes have happened?
Clinical guidelines, patient engagement, physician engagement, and research are all timeless goals. But they may require specificity and some shifts. Identifying what’s truth and accommodating necessary changes ensures that your registry will still point in your organization’s North Star, with a shared set of priorities. Documenting this will help to assess opportunities and minimize distractions, even when those distractions seem monumental.
- What output will help you reach these priorities and goals? In other words, your goals should define what’s visible in your registry. What do your clinical, scientific, and patient communities need to achieve these aims. The reports, dashboards, workflows, and predictive calculators and tools in your registry should deliver the insights your stakeholders need to take action.
- Your registry’s outputs drive how the data should be combined and adjusted. This includes your measures and cohorts, peer groups, benchmarks, and more. How will you measure what matters? What elements and assets need to be combined in order to support those measures?
- Finally come the data. What individual data elements do you need? How will you collect that data securely, efficiently, and accurately?
This process isn't something to do only once or only during implementation. It is something to document and revisit every time there is a shift in your specialty or practice. Doing this will help ensure that the changes and adjustments to your strategy and registry continually serve both your people and your priorities.
Step 2: Use Trusted Data and Advanced Analytics to Address Critical Issues
Addressing new and emerging health issues requires rapid measurement and quick discoveries.
It involves having the right framework that involves two things:
- A comprehensive data collection strategy that ensures you have all the right data.
- Advanced registry analytics that help you answer all the right questions.
Registry Data Collection Strategy
Different data sources supply different information to ultimately evaluate a health issue. Most registries rely on clinical data from the EHR. But most data sources – EHR data especially – has inherent limitations. Clinical data alone often doesn’t provide all of the information needed to fully understand a health issue.
This means registries that rely on EHR or clinical data alone will have data gaps. Yet solving some of the most intractable issues in healthcare will require innovative methods of blending data to deliver a complete view of a patient population. In fact, multiple studies have shown that nearly 80% of health outcomes are attributed to what happens beyond the walls of a health system.
You can address these gaps with a comprehensive data acquisition strategy that leverages not only at EHR data but also administrative datasets, claims datasets, and governmental datasets. You can also use specialized case report forms, provider and patient surveys, and other methods for collecting patient-sourced data.
Collecting data in which you're able to assess root causes of health issues by having all sorts of associated factors that are at play will also enable you to effectively pursue data-driven interventions to improve outcomes and improve care delivery by our providers.
Advanced Registry Analytics
Once you start collecting data, you need to be able to use it to answer the right questions. Enter advanced analytics.
In healthcare, questions seem endless – especially with any emerging issue or research priority. These questions fall into four common types, and there are specialized analytics to address each.
- What happened? Descriptive analytics answers this question by examining data from the past and producing dashboards around trend or benchmarking data. The uses of descriptive analytics can be very limited, especially for healthcare stakeholders who ultimately want to influence health outcomes and have foresight into what might happen in the future.
- What is likely to happen next? Predictive analytics answers this question using modeling and forecasting techniques. Predictive analytics can have limitations, as it is based on determining what will happen if all conditions remain the same, and healthcare leaders often want to understand what will happen after novel interventions or actions.
- What will the effects be if we take some type of action? Prescriptive analytics answers this question using machine learning and numerous inputs to suggest a course of action. For example, decision support calculators use real-time patient data and prescriptive analytics to determine optimal treatment courses based on expected outcomes and likely complications.
- What do we need to explore further? Discovery analytics answers this question using machine learning to analyze raw data, which allows it to determine interconnections, patterns, and outliers that warrant further exploration.
Step 3: Leverage a Flexible Data Infrastructure to Pivot at Any Time
Your new aims, data strategy, and analytics approach can come to life with flexible registry technology and the right registry partner. Modern clinical data registries are designed to evolve as your needs change. Your clinical data registry technology should provide you the ability to:
- Ingest data and apply analytics in real time.
- Collect new data fields and add new measures.
- Rapidly design, build, and distribute patient and provider surveys.
- Intelligently make data available and accessible via web-based reports and dashboards.
The Right Registry Technology Makes Discovery Possible
The ultimate purpose of a clinical data registry is to accurately observe, measure, and understand the true realities of healthcare today. Emerging questions and issues, along with the complexities of real-world data, make this more challenging. None of this important work comes easy. But, as Galileo once said, “All truths are easy to understand once they are discovered.”
When it comes to developing and growing clinical registries that address today’s most important questions, the right technology can support this discovery.
How the Largest Hospitalist Group in the U.S. Is Using Data and Analytics to Understand COVID-19
Sound Physicians is a national medical group focused on acute, hospital-based care. Sound is the largest hospitalist and critical care group in the United States, with more than 3,500 physicians and advanced practice providers in hospital medicine, intensive care, and emergency medicine, at more than 250 hospitals located in 36 states.
In addition to its national scale, Sound is also a leader in physician performance and analytics. They rely on an advanced analytic and IT infrastructure and workflow to improve care, manage performance, and monitor trends.
Real-Time Monitoring to Manage COVID-19
Sound has been monitoring and managing COVID-19 since early 2020 when the first cases started occurring in the United States. In addition to ensuring patients receive appropriate care and achieve the best outcomes, the safety and protection of clinicians is top priority.
Sound’s Chief Clinical Officer, John Birkmeyer, M.D., put it this way in a recent interview with the NEJM Catalyst:
“Our major focus in reacting to the COVID-19 epidemic has been to retool our nationwide IT platform to track in real time which patients have COVID in the presence or absence of testing confirmation. That same IT tool not only applies to patients that have COVID but simplifies our team’s approach to be able to concentrate those patients on specific teams and in specific parts of the hospital.”
Leveraging their proprietary charge documentation application, Sound embedded logic into their technology and steps into their clinical workflows to prompt physicians to identify whether the patient is being treated for and/or tested positive for COVID. Physicians are prompted to answer this question, at both admission and discharge, for any patient presenting with a primary diagnosis of coronavirus, respiratory illness, or sepsis without another cause.
This near real-time data drives ArborMetrix analytic and reporting tools, from which Sound users can gather insights to understand the coronavirus impact on daily admissions, bed capacity, resource utilization, provider well-being, and standard acute care, at a local, regional, and/or national level.
As Dr. Birkmeyer told NEJM Catalyst:
“The other advantage of that type of real-time tracking, particularly given all of the fluidity involved in growth in admission rates, is that it allows Sound as a national organization to better keep its finger on the pulse of the epidemic. Specifically by us appreciating what the prevalence is at any one of our sites, it allows us to tailor our support to the hospital sites and to the physician teams that need that most. Sometimes that support focuses on the availability of PPE equipment — and specifically, where we can, backstopping shortages of N95 masks. But that type of support also extends to providing emotional support and well-being services to physicians who are significantly stressed.”
Sound’s coast-to-coast footprint provides them with a nationally-representative database on COVID admissions, treatments, and outcomes across all of their partner hospitals. This expansive data positions Sound with the unique opportunity to help state governments, federal bodies, media, and other stakeholders, understand the national impact of the pandemic, as it relates to virus spread, population impact, and healthcare resource utilization.

This map from April 21, 2020, shows the volume of suspected COVID-19 cases at Sound Physician partner hospitals in the continental U.S.
Nationwide COVID-19 Dataset Drives Research
Additionally, Sound is partnering with academic researchers to use the data in order to understand:
- The impact the COVID-19 pandemic has on hospitalizations for acute medical illness.
- Risk of infection among front-line healthcare professionals.
- Risk factors for adverse outcomes of COVID-19 in hospitalizations.
- Effects of state social distancing orders on COVID-19 hospitalizations.
- Effects of COVID-19 pandemic on stress and burnout in healthcare professionals.
Through their partnership with ArborMetrix, Sound is able to provide their field leadership and users with interactive reporting tools based on near real-time data, to gain key clinical insights as to how COVID-19 is impacting daily admissions, bed utilization, resource utilization, and provider safety. Armed with data and analytics, Sound is positioned to be a leader in the fight against the coronavirus.
If you have any questions about these programs or would like to learn more about our healthcare analytics solutions for physician organizations or contact us here.
Patient Registry Tracking COVID-19: ELSO and Extracorporeal Membrane Oxygenation (ECMO)

As we face the continually evolving pandemic of COVID-19, each day brings new challenges and opportunities to learn from our shared experiences. This education is fueled by the hard work and dedication of individual clinicians, care teams, research institutions, and medical specialty societies around the globe.
Coordinated efforts by medical societies and their members have a real impact on our ability to fight against the pandemic. These organizations support the collection, analysis, and proliferation of data about this virus at a global scale.
A global crisis needs global collaboration for a global solution.
ECMO Is Proven to be Effective Treatment for COVID-19 Patients with Severe Respiratory Distress
COVID-19 (the illness caused by the novel coronavirus) causes severe respiratory distress in some patients. Extracorporeal Membrane Oxygenation, commonly referred to as ECMO, is a treatment that uses an artificial heart and lung to support the body when a person's own organs are too sick to do the job. ECMO itself will not cure a patient’s heart or lungs, but it gives them the time needed to heal.
ECMO has proven to be an effective treatment for COVID-19 patients with severe respiratory distress. In addition to ECMO being an effective therapy in the treatment of severe Acute Respiratory Distress Syndrome (ARDS), it is a recommended rescue therapy in COVID-19 patients in guidelines published by the Society of Critical Care Medicine.
Global ECMO Organization Tracks and Educates on COVID-19
The leading global authority on the use of ECMO is the Extracorporeal Life Support Organization (ELSO). Its global patient registry is the world’s largest source of data on patients receiving ECMO. ELSO typically supports medical research, continuing education, guidelines development, and device surveillance.
ELSO is uniquely positioned to lead education efforts around the use of Extracorporeal Life Support (ECLS) and ECMO as a treatment for COVID-19 patients. Dr. Mark Ogino, president of ELSO, said in a recent video summarizing ELSO’s COVID-19 response:
“We remain a real-time authoritative resource because of ELSO’s global member centers, which provide up-to-date information and data to allow our physicians and scientists to continually edit our recommendations.”
ELSO Registry Tracking ECMO for COVID-19
ELSO provides support to institutions delivering ECLS through the maintenance of a comprehensive registry of patient data. The ELSO Registry tracks the use of ECMO as a life-sustaining therapy in patients with severe cardiac or pulmonary distress. The registry tracks patient outcomes and complications occurring during ECMO for patients across a wide variety of clinical presentations and geographic areas. Registry data is not only used for publication, but also for benchmark reporting so that participating ECMO programs can see how their outcomes compare to average.
ELSO is waiving participation fees for new institutions and sites that would like to participate in these important tracking efforts. Get started here.
ECMO COVID-19 Resources
ELSO has organized resources and collected data from member centers across the world. Each resource shares the lessons learned in the parts of the world most affected by COVID-19.
Top ECMO resources for COVID-19:
- ECMO in COVID-19
- ELSO’s guidance on ECMO in COVID-19 (available in 11 languages)
- ELSO Blog: COVID-19
ELSO’s coordination and leadership allows physicians and care teams working in areas hit hardest by the pandemic to share best practices, clinical guidelines, and recommended clinical indication and contra-indication for ECMO. This type of open collaboration helps improve care for all.
In addition, ICUs in Italy and China have released checklists aimed at limiting exposure to the virus by physicians during intubation. The coronavirus can spread through aerosol and moisture droplets, so it is especially important to limit physician exposure before, during, and after ECMO.
Global Study on ICU Management of COVID-19 Patients
One of the most important projects being undertaken by ELSO centers and physicians is the Extracorporeal Membrane Oxygenation for Coronavirus 19 Acute Respiratory Distress (ECMOCARD) Study. ECMOCARD is a study coordinated by the Asia-Pacific Chapter of ELSO with 104 participating hospitals across 5 continents and 26 countries.
ECMOCARD is currently the only global study to gather data on the ICU management of the sickest and most vulnerable COVID-19 patients. The ECMOCARD study will be an invaluable resource to manage the treatment of COVID-19 patients. In a time when so much is unknown, the value of rigorously documenting and disseminating the lessons learned every day is of immeasurable value. In the words of ELSO’s founder Dr. Robert Bartlett, “Our most important weapon in this crisis is data to predict and plan – on a global scale.”
At a time when each day bring new and unique challenges, it is more important than ever to collect, document and share experience globally; for in an increasingly interconnected world only through cooperation and solidarity can we overcome a global pandemic.
To stay up to date on the latest information regarding the use of ECMO for COVID-19, visit https://www.elso.org/COVID19 and follow ELSO on Twitter at @ELSOOrg.
If you have any questions or would like to learn more about clinical data registry software, contact me at lleon@arbormetrix.com.
Top Examples of Quality Improvement through Clinical Registries

Achieving real-world quality improvement in healthcare delivery benefits everyone. Patients have improved outcomes and a higher quality of life. Physicians use evidence-based insights to make decisions and advance care. Hospitals and payers achieve efficiencies and greater savings.
This win-win-win of quality improvement can be achieved locally with the right infrastructure, data, processes, and people involved. But what happens when many institutions come together to collaborate, share data, and learn from one another?
Improvements are achieved at greater scale. More people benefit. Today's biggest issues facing patient safety and outcomes are addressed.
Let’s look at the top examples of quality improvement at scale and how they achieved it: One from a regional surgical quality collaborative and another from a national network of pediatric hospitals with cardiac ICUs. Both use healthcare analytics and clinical registries to achieve significant results.
Example 1: Post-surgical Patient Opioid Consumption Drops by 50%
The opioid epidemic is a top priority for physicians and healthcare providers across the continuum of care. This is especially true in surgery. Surgeons prescribe 10% of opioids across the United States, and research shows “the vast majority of these pills are not used, potentially leading to opioid dependence, misuse, or diversion into the community." [1]
With this issue top of mind, in partnership with Michigan OPEN, the Michigan Surgical Quality Collaborative (MSQC) successfully launched a program to reduce the number of opioids prescribed to surgical patients over a 16-month time frame. [2]
The project involved researchers at Michigan OPEN developing evidence-based prescribing guidelines for five operations, based on MSQC-collected data on patient-reported opioid consumption after surgery. MSQC’s registry collected opioid prescribing, consumption, and satisfaction data for more than 10,000 patients over several months. They transformed this data into knowledge and defined and disseminated new opioid prescribing guidelines. Then they continued to collect data, and track and analyze outcomes.
Did the new guidelines make a difference? The results published in the New England Journal of Medicine were clear. They found:
- A 30% decrease in post-operative opioid prescribing.
- A 50% decline in post-operative opioid consumption.
- No change in patient-reported satisfaction with care and pain in the week after surgery.
How did they achieve it?
Reaching these outcomes required MSQC’s deliberate planning, coordination and collaboration, and information and data sharing.
Data-Driven and Patient-Centric Program Design
MSQC organized this initiative and leveraged their infrastructure exceptionally well:
- They used existing research and new data to understand the problem and inform the program.
- They used real-time analytics to accelerate findings.
- They included patient-reported outcomes and satisfaction after surgery.
Their post-surgical opioid prescribing reduction program is best summarized an excerpt from a recent paper:
“Opioids prescribed after surgery have gained national attention for their role in the escalating opioid epidemic.14, 15 To learn about this problem, the MSQC team sought feedback from the collaborative sites, reviewed scientific and practice‐based research, and solicited patient experiences. With this knowledge, the MSQC clinical leadership designated postsurgical opioid prescribing as a quality improvement focus area with high priority. The coordinating center team added additional data collection variables to the MSQC data platform to determine the amount of opioids prescribed after surgery. They also added patient‐reported opioid consumption, and other patient‐reported outcomes including satisfaction with care and postoperative pain. Data were collected on a subset of patients undergoing five of the most commonly performed operations in the MSQC database.” [2]
Flexible and Robust Data System
MSQC’s clinical data registry on the ArborMetrix platform plays a central role in all of its quality improvement programs.
Along with its community 70 hospitals, MSQC uses real-world data to focus relentlessly on achieving real-world quality improvement and make Michigan the best place for surgery in the country. Their approach and proven results have made them a national example of how to advance care and reduce costs using high-integrity data. [3]
The post-surgical opioid prescribing reduction program is no different. For this initiative, MSQC leveraged registry technology that:
- Captures patient feedback after surgery and connects longitudinal data to specific procedures.
- Provides real-time data in reports to enable a rapid research cycle. This allowed them to know early on whether the new guidelines were working right away. They didn’t have to wait a year or longer.
- Easily supports the addition of new data collection variables — both from clinical sources and patient-reported outcomes.
- Flexibly allows for the creation of new reports and dashboards.
Physician Engagement and Performance Feedback
Another central part of MSQC’s model is sharing performance feedback with surgeons and sites through the registry, and giving participants the tools they need to be successful.
- The registry’s real-time, risk-adjusted reports give personalized performance feedback at the hospital and surgeon level. Participants can easily view their performance in comparison to other member institutions, drill down into specific use cases and patients for deeper evaluation, and export the full dataset for further analysis and research.
- MSQC’s tool kits provide a “comprehensive ‘one-stop’ information hub” to participants to simplify and disseminate program guidelines.
Example 2: Critical Pediatric Cardiac Deaths Decrease by 24%
Nearly 40,000 infants born in the United States each year have some form of congenital heart disease (CHD), making it the most common birth defect affecting 1 in every 110 babies.
Pediatric cardiologists and researchers have improved outcomes considerably over the past few decades. Yet many children still experience significant health issues over the course of their lifetimes, according to Michigan Medicine C.S. Mott Children’s Hospital. [4]
New approaches are necessary to make the next leap in CHD care.
A group of physicians and researchers, led by Jeffrey Anderson, M.D., M.B.A., Michael Gaies, M.D., M.P.H., and Sara K. Pasquali M.D., M.H.S., organized Cardiac Networks United to address these challenges. Member institutions span more than two thirds of all hospitals caring for congenital heart patients in the United States. [5]
The Pediatric Cardiac Critical Care Consortium (PC4) is one of five founding organizations of CNU. PC4 aims to improve the quality of care for pediatric heart patients through transparent data sharing that allows hospitals to evaluate their own outcomes and learn best practices. [6]
Their efforts are proving effective and the results are outstanding.
Eighteen hospitals significantly reduced mortality and improved care for children with critical heart conditions, according to a paper published in the December 2019 edition of the Journal of the American College of Cardiology. The study analyzed more than 19,000 hospitalizations that included cardiac surgery at the participating sites in the PC4 registry. [7]
Published results include:
- 24% decrease in postoperative mortality among participating sites between 2014 and 2018.
- 12% reduction in major complications.
- 13% decline in time on a ventilator.
- 5% decrease in length of stay in the ICU.
How Did They Achieve It?
The core principles of collaborative quality improvement drive PC4 and its member community:
- Purposeful collection of specific clinical data on outcomes and practice.
- Timely performance feedback to clinicians.
- Continuous improvement based on empirical analysis.
- Collaborative learning at various events throughout the year.
Member organizations are committed to sharing data and expertise with one another to accelerate discovery and improvement in the care of patients with pediatric and congenital heart disease.
High Quality, Research-Grade Data
In order to achieve the level of trust and accuracy needed for collaborative quality improvement, PC4 puts a huge emphasis on the quality of the data in its registry. It focuses on three key aspects:
- Streamlined data collection.
- Immediate data quality feedback.
- Routine site audits.
Real-Time Performance Feedback to Clinicians
PC4’s registry provides 24/7 access to real-time data to be used for local quality improvement. Participating hospitals use the registry to support their own physicians and care teams. Importantly, PC4 offers access to unblinded center data to facilitate identification of top performing hospitals and stimulate collaboration among sites to improve patient outcomes.
Tailored Analytics
Another key aspect driving PC4’s success are the powerful analytics behind its registry. Participants have access to risk- and reliability-adjusted comparative analyses on quality metrics selected by the consortium.
Recently of note, ArborMetrix helped develop a program-level risk adjusted metric on post-operative mechanical ventilation, measuring quality of care from the time a patient enters the OR to the end of their critical care period(s). This metric is part of PC4’s initiative to liberate children from the ventilator.
Registries Make it Possible
Organizations like PC4 and MSQC are the best examples of quality improvement through clinical registries. They show what is possible when you make a clinical data registry rooted in data science your hub of information to support collaborative data sharing, learning, and progress.
Top 3 Takeaways from the 2020 CMS Quality Conference

Last week, the Centers for Medicare and Medicaid Services (CMS) held its annual Quality Conference in Baltimore, MD. More than 3,000 attendees convened to learn and talk about the present and innovative future of quality improvement on a national scale. Leaders from CMS discussed the current administration’s rapidly-evolving quality agenda.
CMS Administrator Seema Verma outlined key priorities including:
- Establishing clear and reasonable standards and quality measures, “the rules of the road”.
- Strengthening the government’s oversight of said standards.
- Promoting “transparency, competition, and consumer choice” by sharing more information with the public.
- Modernizing “quality improvement efforts for all” via advances in data analytics and technology.
Three themes clearly emerged.
The Future of FHIR is Bright
CMS is serious about harnessing FHIR to ease the reporting burden for stakeholders. They have taken concrete steps toward a FHIR-based connectivity standard over the past year.
CMS is envisioning a centralized submission solution for quality reporting. This single, FHIR-based CMS receiving system will do all the calculations and exchange data and results with the appropriate quality programs. FHIR will enable future submissions to CMS be less complex. A successful pilot connection with Cerner earlier this year gives CMS confidence in its vision.
This move means great things for medical specialty and patient registries in particular. ArborMetrix uses a FHIR-based infrastructure to promote industry standards and support interoperability. This provides flexibility, efficiency, and scalability for the future. Organizations that focus on interoperability among their source data systems and data assets will have a tremendous advantage and unparalleled information access as initiatives like the one above – and others involving EHRs – are fully in place.
The Voice of the Patient Will Be Heard
Dr. Michelle Schrieber, CMS Director for Quality Measurement and Value Based Incentives, described the addition of patient-reported outcome (PRO) measures to the “Meaningful Measures 2.0” framework and the Merit-Based Incentive Payment System (MIPS).
Patient-reported outcome measures not only unleash the patient voice, but also minimize the burden in data collection. At a time when CMS is looking to reduce the number of MIPS measures overall, they are also looking to increase the number of PRO measures. This reflects their overall shift from process to outcomes measures that lean more heavily on the patient.
ArborMetrix believes patient data is invaluable. But using it requires the right strategy and healthcare analytics technology to be sustainable. PRO response rates are highest when used in shared decision making or when you empower the patient to view and engage with their own data.
Overall, it is clear CMS’s focus on PRO data will benefit organizations who have the technical and nuanced experience in longitudinal patient follow up.
Digital Quality Measures Will Simplify Everything
Representatives from CMS made the bold claim that by 2030, all quality measures will be fully digital. This means clinicians will not have to take time away from patient care to comply with quality programs. This is major progress.
CMS encouraged conference attendees to imagine a world where quality data is seamlessly transmitted from EHRs. This future state is exactly what is needed. Clinicians will finally be unburdened and everything they have to do will blend easily into their workflow—what they have spent years asking for.
ArborMetrix welcomes this transition, as it will amplify our medical specialty society partners’ goals to continue to go beyond MIPS when defining their strategic registry goals, measures, and programs. Our partners who have leveraged their registries to do the most impactful work, harness the collective power of their membership to embark on novel research efforts and quality initiatives that save lives. We look forward to helping medical specialty societies clarify their clinical data registry strategy and continue to improve patient outcomes.
The 2020 CMS Quality Conference was a whirlwind of data, presentations, and bold goals. The future of quality improvement that keeps patients at the forefront, embraces FHIR standards, and make QI compliance simple for clinicians, is one that I am really fully ready to embrace.